
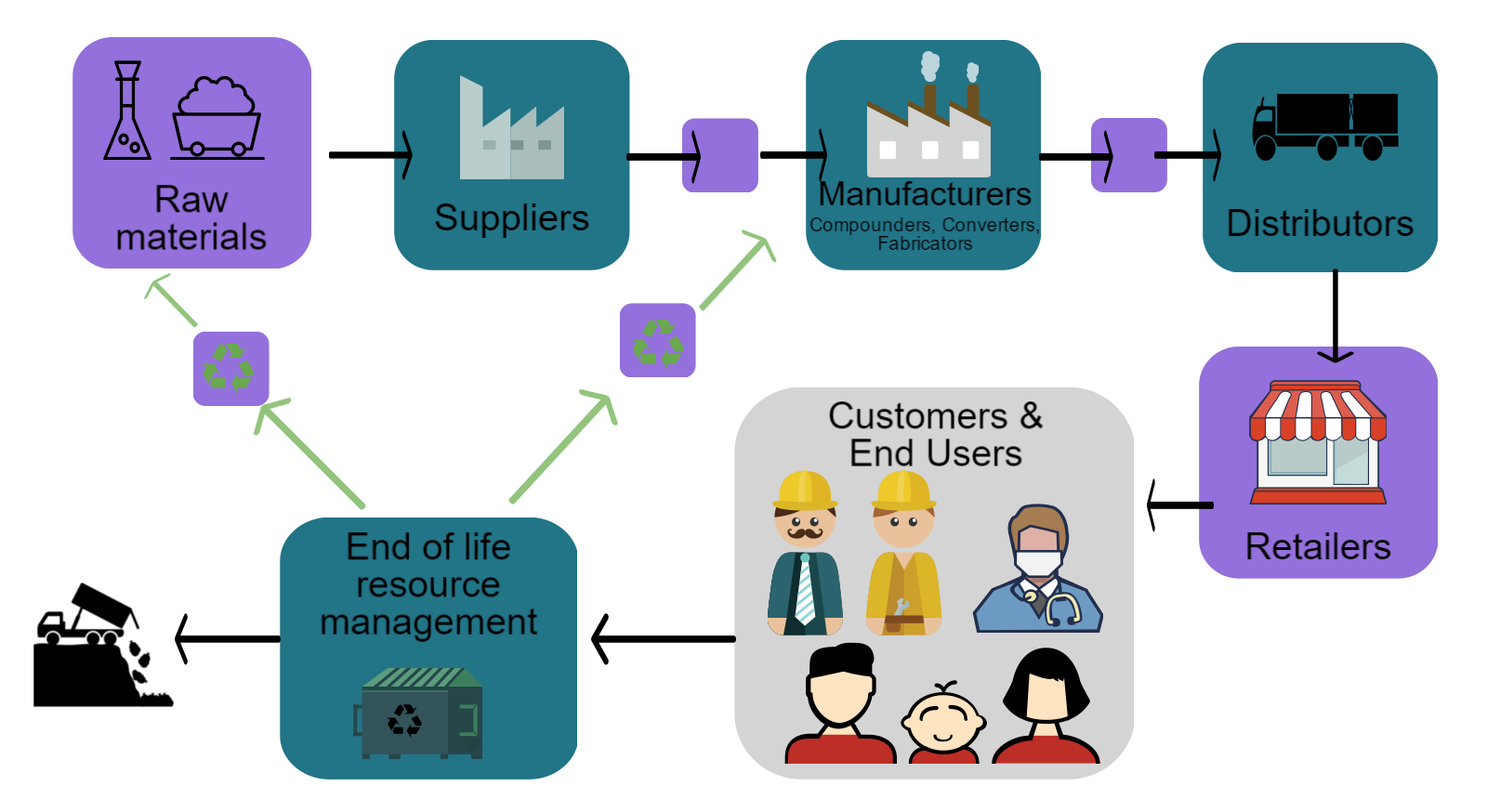
Manufacturing Process
Manufacturing polyvinyl chloride (PVC) is a three-step process, described below. Alternatively, watch our short video for an overview of the process and the manufacturing supply chain.
Step 1 - Producing ethylene dichloride (C2H4Cl2)
Chlorine is extracted from sea salt via electrolysis, and ethylene is derived from hydrocarbon raw materials. These are reacted to produce ethylene dichloride (1,2-dichloroethane).
C2H4 + Cl2 = C2H4Cl2
ethylene + chlorine = ethylene dichloride
Step 2 - Producing Vinyl Chloride Monomer (VCM)
The ethylene dichloride is then decomposed by heating in a high temperature furnace or reactor.
C2H4Cl2 = C2H3Cl + HCl
ethylene dichloride = vinyl chloride monomer + hydrogen chloride
The hydrogen chloride is reacted with more ethylene in the presence of oxygen (a reaction known as oxychlorination). This produces further ethylene dichloride. The resultant ethylene dichloride is decomposed according to the above equation, and the hydrogen chloride is again returned for oxychlorination.
2HCl + C2H4 + ½ O2 = C2H4Cl2 + H2O
= C2H3Cl + HCl2 + H2O
The overall reaction can be shown by adding together the above equations:
2C2H4 + Cl2 + ½ O2 = 2C2H3Cl +H2O
ethylene + chlorine + oxygen = VCM + water
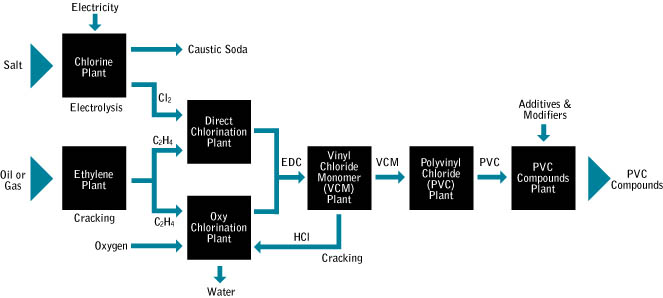
Step 3 - Manufacturing polyvinyl chloride (PVC)
PVC is made using a process called addition polymerisation. This reaction opens the double bonds in the vinyl chloride monomer (VCM) allowing neighbouring molecules to join together creating long chain molecules.
nC2H3Cl = (C2H3Cl)n
vinyl chloride monomer = polyvinylchloride






