The Vinyl Council of Australia represents the PVC/vinyl value chain in Australia.
We're working to advance the sustainability of PVC products and the industry here by encouraging our members to implement product stewardship and best practice manufacturing.
Choose PVC products from companies signed up to the PVC Stewardship program or accredited as Best Practice PVC.
In the PVC industry, but not a member?
Contact us now to find out how we can help you.
Read more news
Latest news
-
Vinyl Council launches updated Best Environmental Practice PVC product verification scheme
2023-05-29 00:42:05Following extensive consultation with stakeholders, the Vinyl Council of Australia (VCA) has launched its updated ‘Best Environmental Practice PVC (BEP)…Read More -
Call for papers for PVC 2024 conference extended till 31 May 2023
2023-05-09 00:01:09Prospective authors are invited to submit abstracts of up to 300 words for consideration for the program for the IOM3's…Read More -
Course materials from Vinyl Council fire behaviour course available for purchase
2023-05-03 01:34:29The Vinyl Council of Australia successfully held its 4-day Hazards from Fire: Quantification of Fire Behaviour Fire Retardancy and Fire…Read More
Upcoming Events
-
VCA AGM 2023: Celebrating 25 years!
2023-05-23 01:52:51The VCA will celebrate 25 years since its inception at this year's Annual General Meeting (AGM) & Awards Dinner, which…Read More -
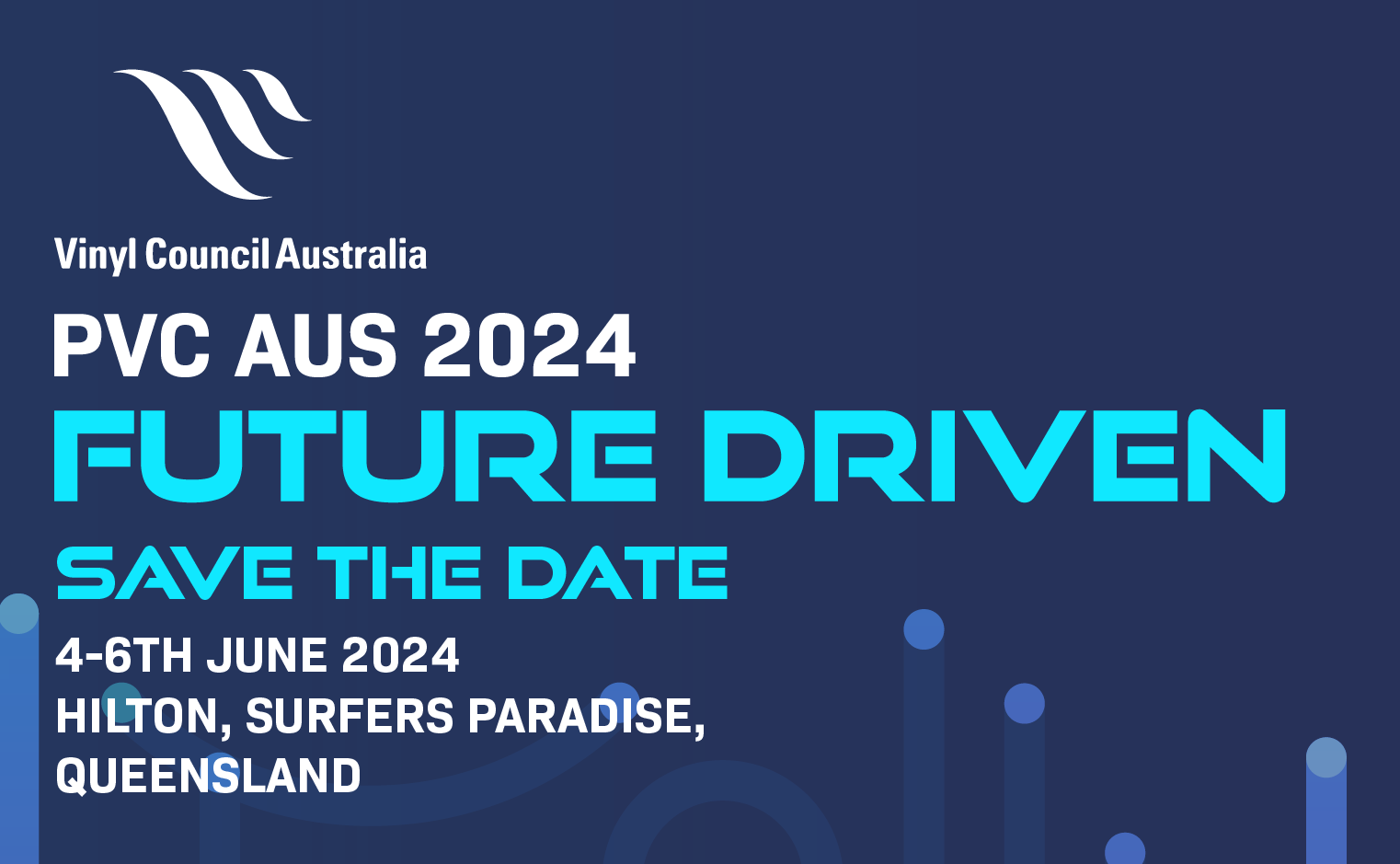
PVC AUS 2024: Future Driven
2023-05-16 00:43:23PVC AUS 2024: Future Driven on the Gold Coast, Queensland. Dates: 4-6 June 2024. The fourth biennial PVC AUS conference…Read More
We recognise the continuing connection of Aboriginal and Torres Strait Islander peoples to Country and acknowledge the Boon Wurrung people of the Kulin Nation on whose unceded lands our office is sited. We pay our respect to their Elders, both past, present and emerging.